40 fda structured product labels
Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics... A dataset of 200 structured product labels annotated for adverse drug ... The Structured Product Labels (SPLs), the documents FDA uses to exchange information about drugs and other products, were manually annotated for adverse reactions at the mention level to ...
What is Structured Product Labeling (SPL)?, HL7, FDA, Regulatory ... Structured Product Labeling (SPL) is a standard document approved and issued by Health Level Seven (HL7) to exchange information related to product and facility. It is used as a base for Regulatory guidance document in exchange for product labeling content. SPL ensures control over critical product information that has led to a standard for product labeling. It is adopted by the Food and Drug ...

Fda structured product labels
Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. [1] The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug. Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL... Structured Product Labeling | Consumer Healthcare Products ... - CHPA In June 2009, the U.S. Food and Drug Administration (FDA) issued a guidance, "Providing Regulatory Submissions in Electronic Format — Drug Establishment Registration and Drug Listing" on its expanded requirements for submissions in the Structured Product Labeling (SPL) format.
Fda structured product labels. FDA SPL - Structured Product & Drug Labeling Composition Process | Reed ... Structured product labeling for both prescription and over-the-counter (OTC) drugs must incorporate an overview of the scientific information needed for the correct and effective use of the drug. The labeling is broken up into sections including explanations for use (prescription drugs) or purpose (OTC drugs), adverse effects, and more. Structured Product Labeling (SPL) Terminology Files The NCIt-SPL terminology files provided here support the cooperative efforts of the Food and Drug Administration (FDA) and the National Cancer Institute's Thesaurus (NCIt) to develop terminology that facilitates the processing and review of SPL Terminology Files data. The efforts are described more fully on the Structured Products Labeling web ... Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The openFDA... NSDE | FDA - U.S. Food and Drug Administration CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for...
Structure Product Labeling / Monograph (SPL-SPM) Submissions The FDA uses SPL documents to exchange information elaborating upon various product-related topics. Structured Product Monograph. In 2016, Health Canada announced that the SPL will be considered a Structured Product Monograph (SPM), and it should be submitted in an electronic format. Ensuring the same, the Agency has released guidelines and ... FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently... PPTX Structured Product Labeling Overview - United States National Library ... Content of Labeling Product Data Elements Product Name Dosage Form Route of Administration Ingredient (active/inactive/adjuvant) DEA Schedule Product characteristics (color, shape, size, etc…) Packaging Marketing Information (category, status, start and end dates) Representative samples of carton/container labels MTHSPL (FDA Structured Product Labels) - Statistics FDA Structured Product Label imprint attribute for shape text: 18077: BLA: Therapeutic Biologic Applications number for the MTHSPL drug: 15324: NDA: New Drug Application number for MTHSPL drug: 11751: DCSA: Controlled Substance Act designation code (e.g. 0,2,3n) 7193: MARKETING_EFFECTIVE_TIME_HIGH:
SPL Xforms | FDA - U.S. Food and Drug Administration To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ... Extracting drug indication information from structured product labels ... These electronic drug labels follow the SPL (structured product labeling) standard that enhances their machine readability. 8 16 17 However, SPL only provides a structure to separate the drug label into sections (eg, Clinical pharmacology, Indications and Usage, Contraindications, Precautions, and Adverse reactions), and the content of the ... Structured Product Labeling (SPL) | Data Conversion Laboratory - DCL Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard. Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... To understand the impact of SPL labels for current drug knowledge management and CPOE system implementation, this paper investigates (1) if SPL labels are sufficient as an exclusive source for drug information for e-prescribing systems today; (2) if SPL labels can be used directly in conjunction with other knowledge sources.
Structured Product Labeling Improves Detection of Drug-Intolerance Issues Introduction and Objective. The HL7 Structured Product Labeling (SPL) standard 1 implemented by the FDA uses the HL7 Reference Information Model (RIM) 2 to represent the chemical and physical nature of medical products and their safe and effective use. While not all of this content is available today, we enrich the 3704 available SPLs with knowledge from the SPL terminology sources, including ...
Package Type | FDA - U.S. Food and Drug Administration In this section: Structured Product Labeling Resources Structured Product Labeling Resources Risk Evaluation and Mitigation Strategies (REMS) SPL Resources
PDF Structured Product Labeling Implementation Guide for FDA Drug ... SPL Implementation Guide for FDA Drug Establishment Registration and Drug Listing v1.0 3 Terminology: None . SPL location: This information is in the beginning of the SPL file.. XML details: The instructions at the start of SPL are the same for every SPL document (the encoding set is dependent on the character encoding used in the SPL) and are in the following form:
MTHSPL (FDA Structured Product Labeling) Source Information Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose
UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Synopsis Health information suppliers can download drug labels in SPL format from DailyMed. Drug labeling on DailyMed is the most recent submitted to the FDA and currently in use. Sites Consulted. FDA Data Standards Council. Rockville (MD): Food and Drug Administration (US). Structured product labeling resources; [cited 2008 Oct 01].
USFDA structured product labeling update - June 2020 In month of June 2020, USFDA updated header of Structured Product Labeling (SPL) XML: Updated xml-stylesheet reference. Updated the schemaLocation of the urn:hl7-org:v3 namespace.
Introduction to FDA Structured Product Labeling - SPL R4 SPL. The Structured Product Labeling (SPL) specification is a document markup standard that specifies the structure and semantics for the regulatory requirements and content of the authorized published information that accompanies any medicine licensed by a national or international medicines licensing authority. UCUM.
Structured Product Labeling Validation Rules - Food and Drug Administration Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ...
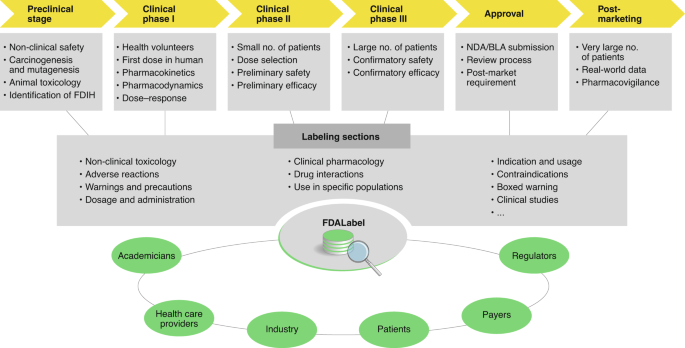
FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents....
DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling Resources page.
Structured Product Labeling | Consumer Healthcare Products ... - CHPA In June 2009, the U.S. Food and Drug Administration (FDA) issued a guidance, "Providing Regulatory Submissions in Electronic Format — Drug Establishment Registration and Drug Listing" on its expanded requirements for submissions in the Structured Product Labeling (SPL) format.
Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL...
Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. [1] The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug.
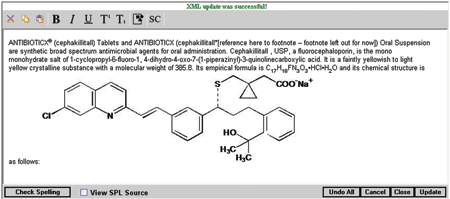

















Post a Comment for "40 fda structured product labels"